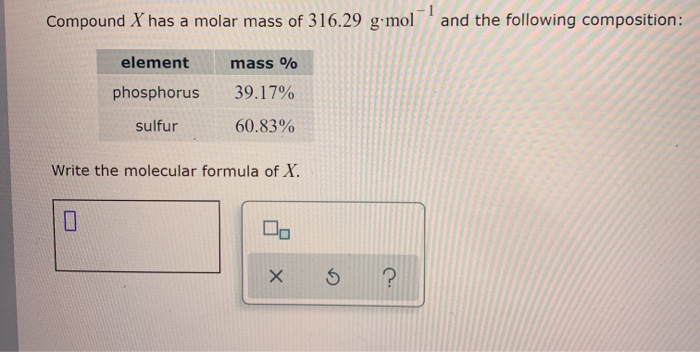
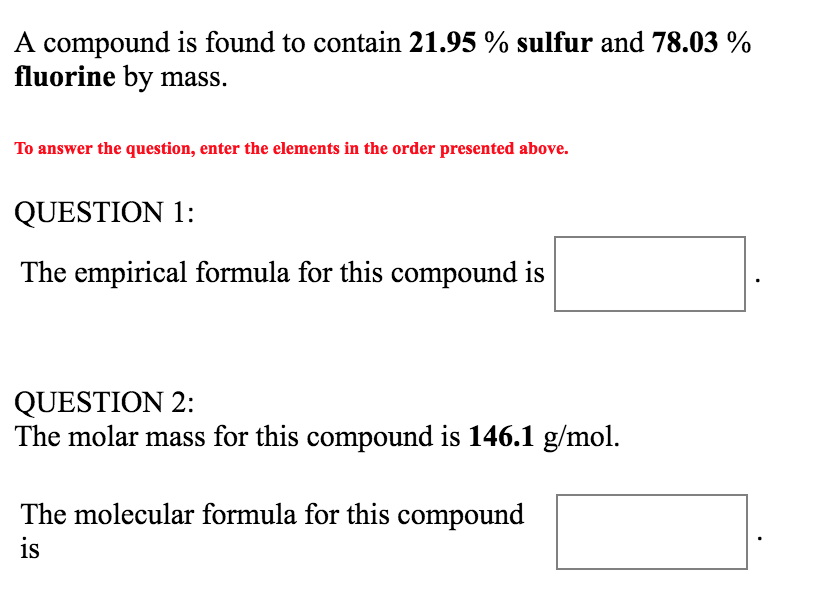
Explanation of how to find the molar mass of Al2(SO4)3 (Aluminum Sulfate).A few things to consider when finding the molar mass for Al2(SO4)3:- make sure you. Molar mass of any element is the sum of atomic weight of that atom which are bonded together in the elemental state. For example molar mass of Oxygen ie O2 is 32 (2 × 16). Molar mass of nitrogen ie N2 is 28 (2 × 14). Thus, molar mass of sulphur ie S8 is 256 (8 × 32).
Let's become familiar with colligative properties and to use them to determine the molar mass of a substance.
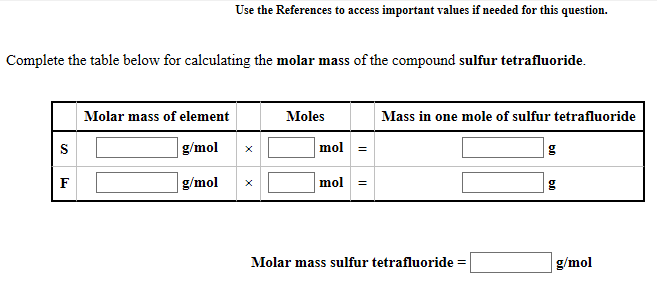
Name: Sulfur Tetrafluoride. Molar Mass: 108.0586. Example Reactions:. SF4 + 3H2O = H2SO3 + 4HF. 3SCl2 + 4NaF = SF4 + S2Cl2 + 4NaCl. ›› Sulfur Hexafluoride molecular weight. Molar mass of SF6 = 1 g/mol. Convert grams Sulfur Hexafluoride to moles or moles Sulfur Hexafluoride to grams. Molecular weight calculation: 32.065 +.6 ›› Percent composition by element. The molar mass of sulfur is 32.1 g/mol, and oxygen is 16.0 g/mol. If you look back at the formula (SO2), you can see that there is one sulfur and two oxygens present. Therefore, the molar mass of sulfur dioxide is 1 × 32.1 + 2 × 16.0 = 64.1 g/mol.
You need this equipment: 600-mL beaker, thermometer, large test tube, 250-mL wide-mouth glass bottle, paper towels, wire gauze, clamp, standard laboratory balance, analytical balance, Bunsen burner, rubber hose, wire stirrer, weighing paper, ring stand, iron ring, two-hole rubber stopper with slit
You need these materials: sulfur, naphthalene
Solutions are homogeneous mixtures that contain two or more substances. The major component is called the solvent, and the minor component is called the solute. Since the solution is primarily composed of solvent, physical properties of a solution resemble those of the solvent. Some of these physical properties, called colligative properties, are independent of the nature of the solute and depend only upon the solute concentration, measured in molality, or moles of solute per kilogram of solvent.
The colligative properties include vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure. The vapor pressure is the escaping tendency of solvent molecules. When the vapor pressure of a solvent is equal to atmospheric pressure, the solvent boils. At this temperature, the gaseous and liquid states of the solvent are in dynamic equilibrium, and the rate of molecules going from the liquid to the gaseous state is equal to the rate of molecules going from the gaseous state to the liquid state.
The phase diagram below illustrates the effect of adding a solute to a pure substance: (view | download)
As demonstrated by the phase diagram above, adding a solute to a solvent lowers the freezing point and raises the boiling point; it also lowers the vapor pressure. The new freezing point of a solution can be determined using the colligative property law:
The change in freezing point is equal to the molal freezing-point constant times the molality of the solution. The molal freezing-point constant used is the constant for the solvent, not the solute.
In this experiment, the molar mass of sulfur will be determined using the colligative property law. The freezing point of naphthalene will be determined experimentally; then a controlled solution of naphthalene and sulfur will be made, and the freezing point of that solution will be determined. The difference in freezing point can be used in the colligative property law to determine the experimental molality of the solution, leading to a calculation of molecular weight. The freezing temperature is difficult to ascertain by direct visual observation because of a phenomenon called supercooling and also because solidification of solutions usually occurs over a broad temperature range. Temperature-time graphs, called cooling curves, reveal freezing temperatures rather clearly. The cooling curve will look like the one below in figure 19.2: (view | download)
In order to minimize supercooling, the solution will be stirred while freezing. To determine the molar mass of a substance, one must simply divide the grams of substance by the number of moles of substance present. All of these values will be determined experimentally.
Procedure:
► A. Cooling Curve for Pure Naphthalene- A large test tube was weighed to the nearest .01 g using a standard laboratory balance. Approximately 15 to 20 grams of naphthalene was added to the test tube. The test tube was weighed again using the standard balance.
- The following apparatus was assembled using the labeled parts: (view)
- The 600-mL beaker was nearly filled with water. It was heated to about 85°C. The test tube was clamped in the water bath as shown in Figure 19.3 above. When most of the naphthalene had melted, the stopper containing the thermometer and stirrer was placed into the test tube. The thermometer was not allowed to touch the bottom or sides of the test tube.
- When all of the naphthalene had melted, the test tube was removed from the beaker of boiling water. The test tube was placed into a wide-mouthed bottle with some paper towels at the bottom. The temperature reading from the thermometer was recorded every 30 seconds. The naphthalene was stirred using the wire stirrer to ensure even freezing. When the temperature remained constant for several readings, the naphthalene was allowed to cool without further temperature readings.
- Using weighing paper and an analytical balance, approximately 1.2 to 1.5 grams of sulfur were weighed.
- The test tube containing the naphthalene from Part A was placed into the water bath from Part A and heated until all of the naphthalene had melted.
- The stopper was removed and the sulfur was poured in. The stopper was replaced and the solution was stirred gently until all of the sulfur had dissolved in the naphthalene.
- The test tube was removed from the water bath and placed into a wide-mouth glass bottle with paper towels at the bottom. The temperature reading from the thermometer was recorded every 30 seconds. The solution was stirred using the wire stirrer to ensure even freezing. When the temperature remained constant for several readings, the solution was allowed to cool without further temperature readings.
- The test tube containing solid solution was handed to the laboratory instructor for proper disposal of chemicals.
Observations:
► A. Cooling Curve for Pure NaphthaleneMass of test tube and naphthalene: 45.93 ± .01 g
Mass of test tube: 32.24 ± .01 g
| Time (s) | Temperature (°C) ± .2°C |
|---|---|
| 0 | 97.4 |
| 30 | 90.2 |
| 60 | 86.2 |
| 90 | 83.8 |
| 120 | 80.8 |
| 150 | 80.6 |
| 180 | 80.4 |
| 210 | 80.4 |
Mass of sulfur: 1.497 ± .001 g (weighing paper was tared)
The sulfur was in powdered form. It was bright yellow in color.
| Time (s) | Temperature (°C) ± .2°C |
|---|---|
| 0 | 100.2 |
| 30 | 94.4 |
| 60 | 88.6 |
| 90 | 85.4 |
| 120 | 82.0 |
| 150 | 78.6 |
| 180 | 77.8 |
| 210 | 77.8 |
| 240 | 77.8 |
Results: (view)
There are two cooling curves required for the results section. The first curve plots time versus temperature of pure naphthalene; the second curve plots time versus temperature of a solution of sulfur in naphthalene. Unfortunately these cooling curves are not available online.
Discussion: The known molar mass of S8 is 256.8 grams per mole. The calculated molar mass of S8 is 290 grams per mole. A percent error calculation can help to measure the accuracy of the experiment. (view)
This error is attributable to several sources of error that were present in this experiment. While the imprecision of instruments is not technically a source of error, it had a particularly devastating effect on this experiment. The imprecision of the thermometer was chiefly responsible for a plus/minus range of 50 grams, which severely restricts the accuracy of the final result. Additionally, the transfer of powdered sulfur from the weighing paper to the test tube may have been incomplete. Some particles of sulfur may have been lost or may have gone unreacted due to the imperfection of the transfer method.
The theory associated with this experiment is the atomic theory of matter. The atomic theory of matter offers explanations for bonding and physical phase. The phase diagram from the introductory section illustrates the difference between pure substance and a solution. The pressure of a solution is lower than the pressure of the pure substance because when a solute is present, the surface of the solution is comprised of solute particles and solvent particles, instead of only solvent particles. There are fewer opportunities for volatile solvent particles to evaporate in a solution. When the vapor pressure of a solution is lowered, the freezing point is lowered. Because the number of molecules of solute has a direct effect on the rate of evaporation, the freezing point depression of a solution is proportional to the molality of the solution.
There are many ramifications associated with this experiment. First, personal experience using colligative equations was gained. Techniques to prevent supercooling were practiced. There are many practical applications of colligative properties. Antifreeze is a solution with a depressed freezing point. The freezing point is lowered by the solutes in the solution as detailed above. Instead of freezing at 0°C, most automotive antifreezes do not freeze until −40°C. In industry, colligative properties can be used to alter the freezing points and boiling points of substances to fit special applications. If necessary, vapor pressures can be lowered, or osmotic pressure can be changed. Solutions can be modified so that any necessary conditions are met.
Questions:
Molar Mass Sulfur
1. For major sources of error in this experiment, consult the second paragraph of the Discussion section.
2. If the thermometer consistently read a temperature 1.2°C lower than the correct temperature, there would be no change in the molar mass calculated. The calculation of the molar mass depends solely on the change in freezing temperature. As long as the thermometer was consistently wrong, the change in temperature would be the same regardless of error. Thus, there would be no net impact on the final molar mass.
3. If the freezing point of the solution had been incorrectly read .3° lower than the true freezing point, then the observed ΔTf would have been greater than it should have been. This would have caused the calculated molality to be too high. If the calculated molality were too high, then the number of moles present as calculated would be too high. An exaggerated number of moles would cause the calculated molar mass to be too low, as the same weight of the substance would be distributed against more moles of that substance. The substance would seem lighter than it is.
4. The following aqueous solutions are arranged in order of increasing freezing points, based on the premise of ion dissociation:
5. In a .050 m solution of NaCl in .150 kg of water, the mass of NaCl is as follows: (view)
6. (view)
7. In order to determine the molar mass of 2.00 g of para-dichlorobenzene in 50.0 g of cyclohexane using colligative equations, the following equations must be employed: (view)
Conclusion: The purpose of this experiment, to become familiar with colligative properties and to use them to determine the molar mass of a substance, was achieved successfully. The experiment was not very accurate, but it was still successful in terms of the original purpose.
Molar mass of SF6 = 146.0554192 g/mol
Convert grams Sulfur Hexafluoride to moles or moles Sulfur Hexafluoride to grams
Molecular weight calculation:
32.065 + 18.9984032*6
| Symbol | # of Atoms | Fluorine | F | 18.9984032 | 6 | 78.046% | |
| Sulfur | S | 32.065 | 1 | 21.954% |
Molar Mass Sulfur Trioxide
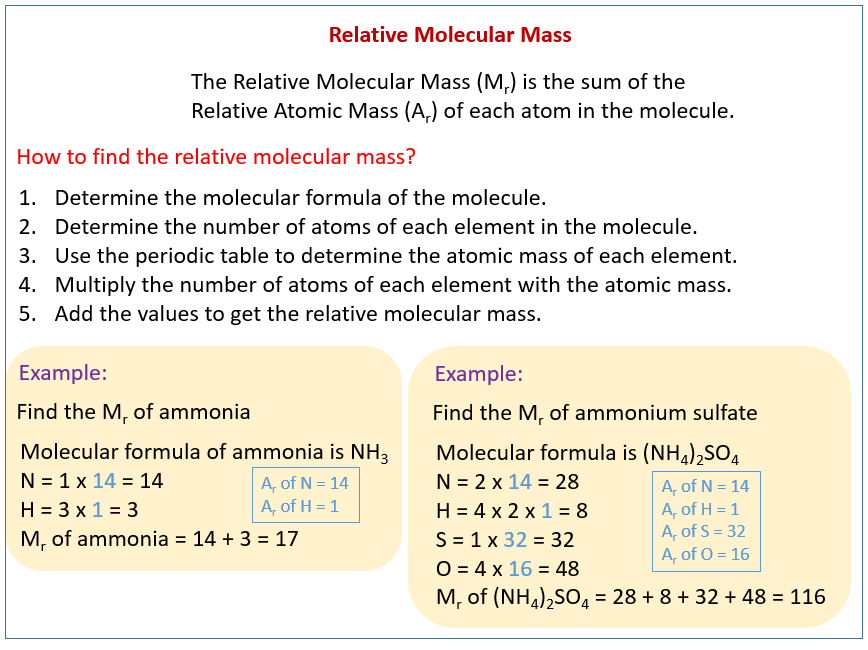
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.

